Summary Novel methods and technology platforms developed Applications
Dynamic Contrast Enhanced Optical Imaging In silico modeling in optical molecular imaging Biomedical Spectral Imaging
Outline
This project aims at estimating neoplasia-related functional and micro-structural parameters thought the modeling and analysis of dynamic optical signals, measured in living tissues. These signals are typically generated as a result of the interaction of the administered biomarker with the tissue in optical molecular imaging applications.
Τhe fact that the dynamic optical effects associated with biomarker’s uptake kinetics (see DCE-OI paragraph) seem to correlate well with the neoplasia grade raises a new scientific challenge defined as to whether it is possible to estimate the values of a list of structural and functional parameters from in vivo measurable (biomarker-induced) dynamic optical characteristics.
As a first step towards this end we have identified a list of biological parameters that are correlated with the neoplasia growth and are determining the agent-uptake pharmacokinetics. On the basis of these findings, a compartmental model has been developed that accounts for all epithelial transport phenomena and predicts the dynamic optical characteristics for any given combination of biological parameter values.
Approach
The three basic steps of the developed method for estimating biological parameters related with neoplasia progress are summarized below
– model-based fitting of dynamic optical signals measured in vivo
- depend on a long list of biomedical parameters and combinations
– global sensitivity analysis
- returns a subset of parameters that are key determinants of the models output
– global optimization approaches (Shuffled Complex Evolution Algorithm)
- returns the value of parameters, when converged to a unique solution
Particularly the global optimization procedures involve
- A regression scheme that utilizes the Euclidean distance between simulated and experimental data as an objective function.
- This function is iteratively minimized and the model parameter values that correspond to this minimization are expected to be the solution of the inverse problem.
It should be noted that the occurrence of multiple extrema makes hard the inverse problem solving in nonlinear optimization.
Findings
 |
 |
|
|
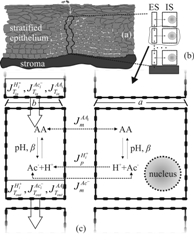
Fig. 1. a) The schematic representation of the cervical stratified epithelium; b) a detail of (a) showing a cell column with the basic extracellular space (ES) and intracellular space (IS) compartments; c) paracellular and transmembrane fluxes of acetic acid and its hydrogen and acetate ions. |
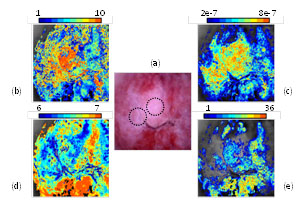
Fig. 2. (a) The image of a high risk cervical epithelium. The circles denote the areas from where biopsies have been collected and diagnosed as High Grade Neoplasia. (b)-(e) Pseudocolor maps of N, b, pHES and ε neoplasia-related parameters respectivelly, as they have been estimated with our method for the first time. Color-coding of the maps corespond to various parameter value ranges. |
Figures 1(b)-(e) illustrate the four maps expressing the value-ranges and the spatial distribution of the four parameters, calculated for every image pixel. Values are increasing from blue to red. Parameter N (b) expresses the number of the epithelial layers and parameter (b) the extracellular volume. These are structural parameters and it is known that both are increasing with neoplasia growth. Quite impressively, the maps show increasing values for both N &b in the vicinity of the (biopsy confirmed) neoplastic areas (circles in a) strongly suggesting the validity of our method. The same applies to figures d & e where two functional parameters are mapped; namely: the extracellular pH and the agent’s paracellular flow barrier (tight junctions). It is known that both of them are decreasing during the neoplasia growth, something that is perfectly predicted by our method.
Impact
It is essential to stress here the fact that the diagnostic importance of the pHES (ES: extracellular space) parameter has only recently been identified and appreciated. According to the so-called "acid-mediated tumor invasion model", there is a strong link of the reduced pHES with the ability of tumor cells to form invasive cancers. Particularly, it has been shown experimentally that H+ flow to peritumoral normal tissue provokes extracellular matrix degradation and normal cell necrosis or apoptosis. At the same time, tumor cells develop resistance to acid-induced toxicity during carcinogenesis. This permits them to spread and invade the damaged normal tissue promoting metastasis. It is therefore expected this parameter to have high predictive value for invasion and therefore can be used for grading and staging of the lesion.
The fact that these parameters are the key determinants of measured/modeled dynamic optical characteristics indicates that they can be estimated concurrently and for every spatial point of the tissue through noninvasive optical measurements.
Conclusion
We introduce a new, noninvasive method for estimating concurrently and non-invasively a set of four biological parameters with proven correlation with neoplasia growth, and hence of undisputed diagnostic value. This is obviously very essential since valuable knowledge will be gained on how functional and structural biological factors are independently or in combination altering during the neoplasia growth, as well as for developing and evaluating novel diagnostic and treatment modalities.
