Summary Novel methods and technology platforms developed Applications
Dynamic Contrast Enhanced Optical Imaging In silico modeling in optical molecular imaging Biomedical Spectral Imaging
Concept and Method
We are pioneers in developing the Dynamic Contrast Enhanced Optical Imaging DCE-OI concept as a means for detecting epithelial neoplasia (see relevant patents and publications going back to 1998). This project started in 1995 and continues in full-force until today.
The method utilizes contrast agents, having the property of lighting-up the altered functionality and structure of the abnormal cells selectively. Specially developed imaging devices measure the agent-induced alterations in tissue optical characteristics as a function of time and at specific spectral bands and for every image pixel.
We have found that in the case of cervical epithelium the obtained dynamic optical data are correlated with the neoplasia grade, thus enabling the unbiased differentiation between neoplastic and non neoplastic lesions as well as between neoplasias of different grades (see clinical studies)
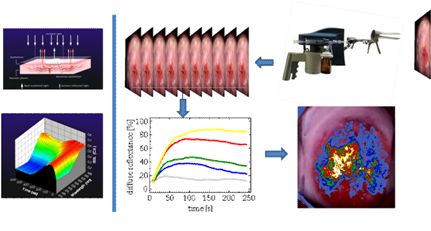
Dynamic optical parameters are calculated for every image pixel (corresponding to several millions of tissue points) and the spatial distribution of their values are making-up the pathology/grade map of the lesion. The various value ranges of these biophysical parameters are directly visualized with the aid of a pseudocolor map, which is overlaid to the real-time color image of the tissue. Red-yellow-white (artificially colored) areas correspond to high-grade lesions that need to be treated or to be followed-up.
Technology and Instrumentation
The Dynamic Spectral Imaging System (DySIS™) was developed to implement Dynamic Contrast Enhanced Optical Imaging in the case of cervical cancer and pre-cancer diagnosis. I designed and supervised the development of three pre-industrial versions and I patented all aspects of this technology. My designs refer to all the mechanical-electronic-electrooptic subunits along with the algorithms and software modules of the DySIS™ imaging device. I also developed the regulatory clearance strategy referring to both CE-Mark and FDA and drafted the relevant applications.
 The basic operational characteristics of the device are outlined below:
The basic operational characteristics of the device are outlined below:
The system embodies a mechanism for the uniform application of a standardised volume of acetic acid solution, which also triggers the initiation of a snap-shot imaging procedure. A plurality of colour and spectral images are captured and stored and from these data the changes in diffuse reflectance (DR) versus time and wavelength are obtained for every image pixel. The experimental data are modelled and analysed and quantitative parameters expressing the degree, duration and extent of the acetowhitening effect are calculated and displayed. The spatial distribution of these parameters makes up the pharmacokinetic map of the lesion, which is visualized with the aid of a pseudocolor scale.
The DySIS™ device produces an information-rich diagnostic signal, enabling the detection and mapping of functional and structural alterations occurring in abnormal cervical epithelial cells during the progress of the neoplasia. The obtained kinetic data have been correlated with the neoplasia grade, in large international clinical trials, where it was shown that DySIS™ offers an unbiased differentiation between neoplastic and non neoplastic lesions as well as between neoplasias of different grades.
DySIS™ performs a rapid (4 minutes), quantitative and side effect-free examination procedure to provide in-vivo topography of the lesion.
The device and the technology eliminate the subjectivity inherent in current diagnostic procedures (cytology and colposcopy) and thus eliminate the extreme burden on health care systems caused by instances of incorrect diagnoses of lesions.
As such, DySIS™ technology emerges as an indispensable tool for the diagnosis, screening and follow up, and for the on-line guiding of biopsy sampling and surgical treatment.
Clinical trials
DySIS has been tested in two independent large, multi-centre clinical studies resulting in three peer-reviewed publications.
1st Clinical Trial
Participating organizations:
Clinical validation is a critical point for any new medical technology. I am honored by the fact that several tenths of high-caliper physicians have worked hard for clinically evaluating my inventions and for providing feedback. Between them I can clearly see Dr. William Patrick Soutter-currently Honorary Reader of Gynecologic Oncology at Imperial College, UK, as the person who supported and inspired me the most. Pat is a legend in gynecologic oncology and everybody respects him as an open minded, brilliant clinician and researcher.
We worked closely together with Pat in all aspects of setting up a fair clinical validation of DySIS, including design of the study, protocol drafting and approval, etc. We also submitted and obtained funding from the U.K. SMART program LOT/031/428 and from the Greek "Praxe-B" program for supporting the clinical study. In total, 529 women were recruited, belonging to either the training or the validation group in the three participating clinics: Hammersmith, St Mary's/London and Alexandra/Athens. In 2009, we published in "Clinical Cancer Research" journal (impact factor 7.5) the results of this study. All eligible members of the validation group (308 women) had an abnormal pap-test and had been referred for colposcopy. The diagnostic performance of DySIS was compared against cytology (Papanicolaou (Pap-) test) and colposcopy, using histology as the "golden standard". According to the protocol, DySIS performed detection and grading of the lesion in an automated basis, solely based on the colorcodes of the DySIS map. In other words there was no interference between the DySIS scan and the operator's assessment.
Traditional colposcopy (operated by experts) missed 37 out of the 72 patients with high-grade disease, achieving a sensitivity of just 49%. The objective and user independent DySIS scan identified an additional 22 patients with high-grade (CIN2+) disease delivering a sensitivity of 79%.
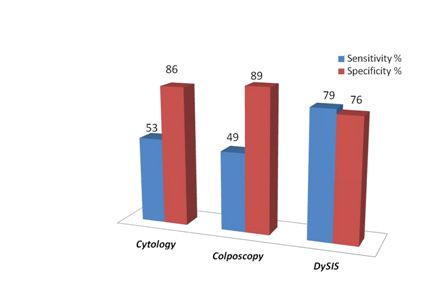
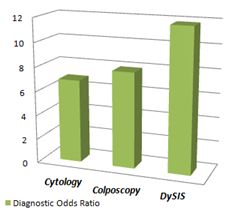
This impressive increase in sensitivity (about 63%) was achieved at a small cost in specificity. However, this is of much lesser clinical importance since a slightly reduced sensitivity would eventually result in taking few more biopsies. The overall diagnostic performance, expressed as the "Diagnostic Odds Ratio", which accounts for both sensitivity and specificity, indicates clearly the superior performance of DysIS over traditional methods.
The findings of this study are summarized in the concluding statement of the CCR article:
"DySIS clearly exceeds the performance of conventional colposcopy even when the latter is operated by experts. It provides an objective assessment and guidance for improving the biopsy sampling accuracy and therefore it is also highly suited for operation by nurses. These characteristics suggest the role of DySIS as a replacement for conventional colposcopy, holding the promise to improve the cost-effectiveness of the diagnostic chain. There is also the potential to apply this very promising technology to screening in low resource settings in which visual inspection with acetic acid is currently under investigation".
2st Clinical Trial
Participating organizations:
End point 1: Assessment of DySIS performance in a typical clinical setting, i.e. with DySIS map being integrated with all other colposcopic indicators
The first trial proved that DySIS offering superior and automated diagnosis can be operated in both colposcopy and screening settings. However the question: "how much the performance would be further improved by combining the performances of both DySIS and colposcopist, remained unanswered". To address this issue, Dr. René Verheijen (currently Prof. of Gynaecological Oncology & head of surgical gynecology Dept, University Medical Center Utrecht (UMCU)) and I took together the initiative to setting up a second clinical trial to be performed in the listed above leading Universities and hospitals in the Netherlands. In 2011, we published the results of this study were published in the BJOG: The International Journal of Obstetrics and Gynaecology (impact factor 3.4). The published results confirmed the findings of the 1st trail. Moreover, it was found that when DySIS is used as an adjunct to conventional colposcopy the combined sensitivity reaches the value of 88% but at a more (as compared with the first study) significant cost in specificity (69%) (all % figures @ 95% Confidence Interval).
End point 2: Comparison of the performance of conventional colposcopy and DySIS in detecting High Grade neoplasia (HG or CIN+) in population infected by Human Papillomavirus, associated with different risks for neoplasia.
Uterine cervical cancer is the second most frequent invasive cancer in women worldwide and is caused by malignant transformation of cervical epithelium induced by high-risk (hr) human papillomavirus (hrHPV) through premalignant stages of cervical intraepithelial neoplasia. It has been established that the most oncogenic virus is the number 16 virus. HPV screening tests are utilizing the same cell sample collected for a pap-test. An abnormal pap-test combined with a high risk HPV-test result is a strong indicator for the presence of high grade neoplasia. The challenge however is whether colposcopy can effectively detect ("harvest") and locate the predicted by the pap-test and the HPV-test neoplastic areas for subsequent treatment or biopsy sampling. Otherwise the predictive value of the screening tests would remain effectively unexploited.
The subject of this 2nd endpoint study was to elucidate whether DySIS is more efficient in "harvesting" more true HG cases than conventional colposcopy, in both HPV 16 and non-16 hrHPV populations. The results of the study were published in second BJOG article in 2012 and referred to sample of 177 women aged 18 years or over.
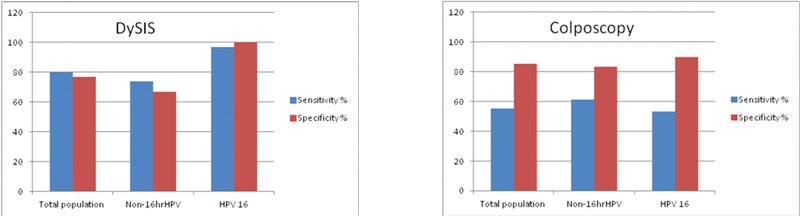
As it can be seen in the histograms, conventional colposcopy misses 45% of the true HG lesions in the entire population of hr-HPV lesions. On the contrary, DySIS displays a much better sensitivity by detecting and locating 80% of the true HG lesions in the same population. The results of DySIS are even more impressive in the HPV16 population where it detects 97% of the true HG lesions at 100% specificity. The performance of conventional colposcopy remains very poor (sensitivity 53%, specificity 90%) in the HPV 16 positive population.
These results suggest strongly that the improved performance of the combined pap-HPV tests are much better exploited by using DySIS in colposcopy, since it effectively harvests the predicted lesions and accurately delineates them, through the DySISmap, for biopsy sampling and treatment.
